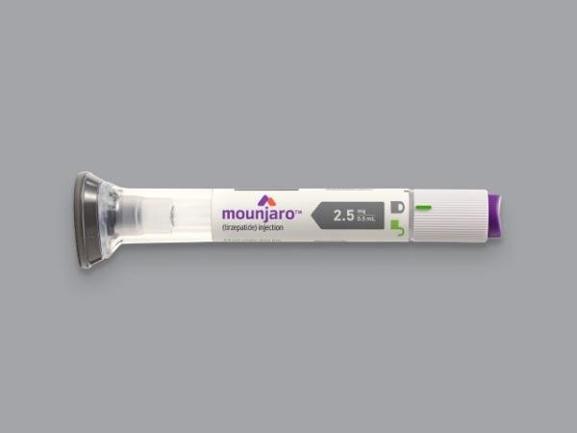
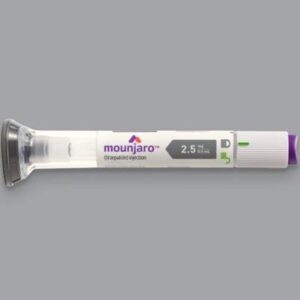
Mounjaro 2.5 mg/0.5 mL pre-filled single-dose pen
Mounjaro® (tirzepatide) is a prescription injectable medication used to help improve blood sugar control in adults with type 2 diabetes. It works alongside diet and exercise and belongs to a new class of medications known as dual GIP and GLP-1 receptor agonists. By mimicking the effects of two important hormones—glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1)—Mounjaro helps regulate blood sugar, improve insulin sensitivity, and may support weight loss.
The 2.5 mg/0.5 mL pen is the starting dose in the Mounjaro titration schedule. It is used for the first 4 weeks of therapy to help the body adjust to the medication and minimize side effects such as nausea and gastrointestinal discomfort.
Dosing and Administration:
Each pre-filled, single-dose pen contains 2.5 mg of tirzepatide in 0.5 mL of solution. It is administered once weekly via subcutaneous injection in the abdomen, thigh, or upper arm. The pen is ready to use—no mixing or measuring is required. After the initial 4-week period at 2.5 mg, the dose is typically increased to 5 mg once weekly, based on patient tolerance and treatment goals.
Storage:
Mounjaro should be stored in a refrigerator between 36°F and 46°F (2°C to 8°C), but it can also be kept at room temperature (up to 86°F or 30°C) for up to 21 days. Do not freeze the pen or expose it to direct heat or sunlight.
Safety Information:
Mounjaro is not intended for use in individuals with type 1 diabetes or to treat diabetic ketoacidosis. It should not be used in people with a personal or family history of medullary thyroid carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). The most common side effects include nausea, vomiting, diarrhea, decreased appetite, and constipation. Serious but less common risks include pancreatitis, gallbladder problems, kidney issues, and a potential risk of thyroid C-cell tumors.


Reviews
There are no reviews yet.